India’s once again facing the spectre of a paralysing disease that had been eradicated, though the World Health Organisation (WHO) has downplayed the potential threat.
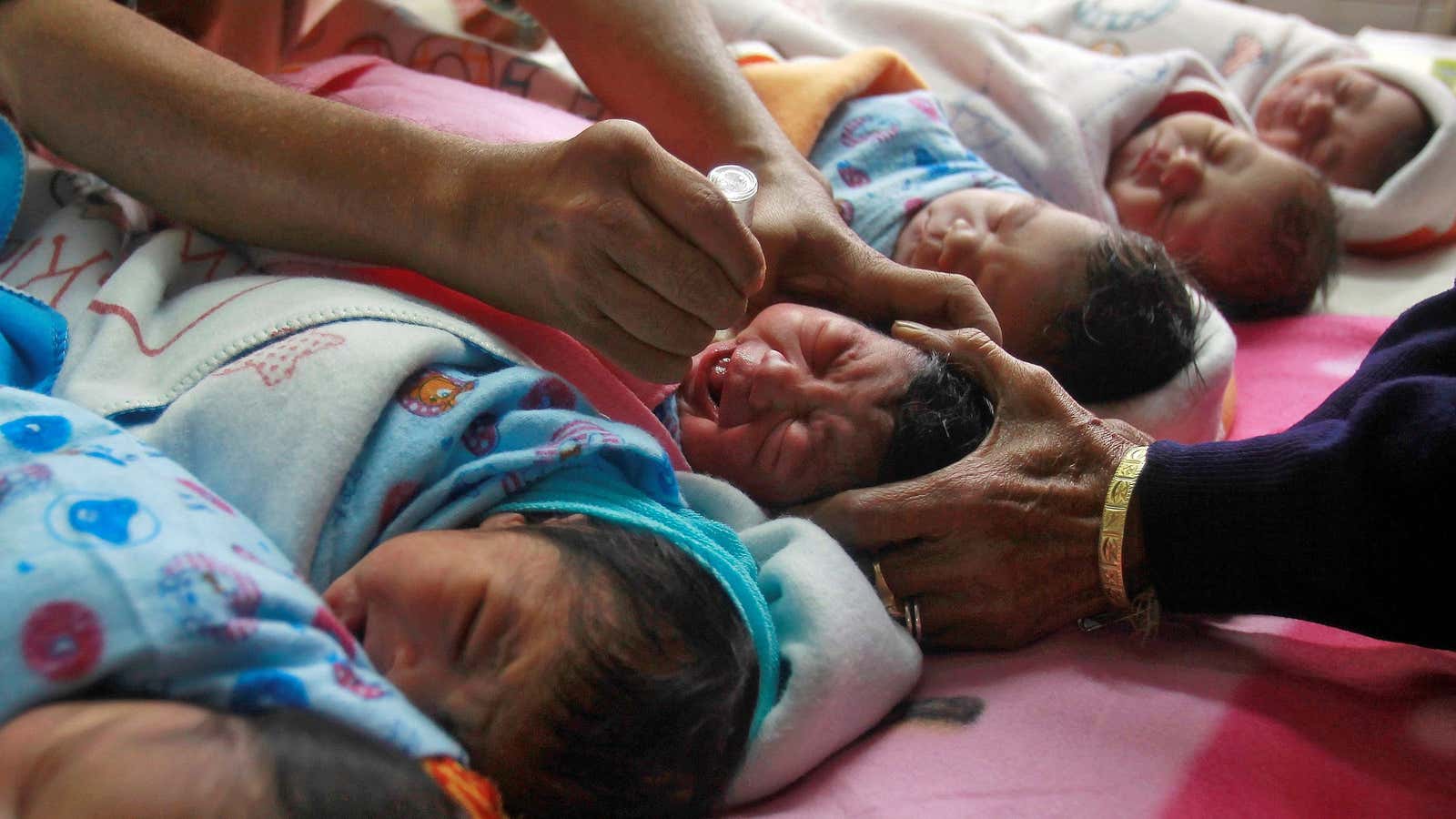
The detection of contaminated vials of type-2 polio vaccine among batches administered to children in some parts of the country has sparked concern in medical and government circles, besides the public.
A private pharmaceutical company reportedly reintroduced the virus in at least 150,000 vials of its oral vaccine, which were then administered to children in the states of Uttar Pradesh, Maharashtra, and Telangana.
In September 2015, the WHO had declared type-2 polio fully eradicated. On the WHO’s advice, India phased out the type-2 virus from its oral vaccines in April 2016 to ensure that the threat of vaccine-derived polio was mitigated.
“The reason why type-2 vaccine was systematically withdrawn was because the wild virus was eradicated, and only harm was being done by the vaccine virus,” said Oommen Kurian, public health fellow at the New Delhi-based think-tank Observer Research Foundation. “When the enemy is absent, there is no case for injuries by friendly fire.”
However, the recent identification of the type-2 strain by government health inspectors in the stool samples of children in Uttar Pradesh has raised fears of a public health crisis.
Virus and vaccine
Polio, a permanently paralysing and sometimes fatal disease, can afflict children if they come into contact with any of the three distinct polio viruses—types 1, 2, and 3. So, from infancy up to six years of age, four doses of vaccination are administered, either orally or through an injection, to immunise them.
The injective method, known as inactivated poliovirus vaccine (IPV), contains only dead strains of the three types of the virus. “IPV does not cause vaccine-derived infection or outbreaks,” Kurian said.
The oral method, however, contains live, but less virulent, strains of the virus and can result in vaccine-induced polio. However, it is extremely rare—about one in 750,000 cases.
“The vaccine virus is a problem because of all the oral vaccine strains, the strain of type-2 is most likely to cause vaccine-associated paralytic polio, which is why it was removed in the first place,” said public health researcher Sylvia Karpagam.
Who’s at risk?
The WHO has, however, downplayed the risk, crediting the high routine vaccination coverage in India.
“The strains reintroduced were of the vaccine virus which is less virulent (compared to the wild virus). Its mutation would have required multiple exposures,” paediatrician and polio researcher Jacob T John agrees. “The risk is low, but it should have remained zero.”
If the virus does turn virulent, the risk would be greater for kids who have received only the oral vaccines from which the type-2 strain was removed in 2016.
“Children who have received three doses of trivalent oral vaccination (given before April 2016) are protected. After 2016, children who received IPV are protected,” said Karpagam.
“The risk will be for kids who had only bivalent oral vaccination (given after April 2016) or no polio vaccine at all, as there is no herd immunity (the resistance of an entire population to a disease) to protect them anymore, because IPV protects only individuals,” Kurian said.
The government has not fully replaced the oral vaccines with IPV because it is more expensive, in short supply, and protects only individuals instead of entire communities.
IPV penetration is also lower among marginalised communities, Karpagam said. “Children residing in slums, conflict areas, orphan children, and children of migrant workers are more at risk.”
Government response
India’s health ministry has said it is ramping up IPV vaccination “in the specified areas to reach out to such children who may have missed IPV”.
“If kids have IPV along with the bivalent oral vaccine, they will be free from any risk. Kids who skip IPV and kids who skip vaccines altogether are at risk,” Kurian said.
Government teams in Telangana and Mumbai are also conducting door-to-door visits to monitor the children who may have received the contaminated vaccine.
After the type-2 strain was found in Uttar Pradesh, the virus was traced to the Ghaziabad-based pharmaceutical firm Bio-Med. Its managing director was arrested and further production was halted.
“Quality control in the pharmaceutical sector has been a matter of concern for India,” Kurian said. “The government should be making sure that trivalent vaccine is not being released by design or by accident. That is exactly what is being done, even if it is being done rather late.”